Wazzup Pilipinas!?
Different ways of coloring a pattern. (Photo credit: Junio, A. O. Colorings of patterns fixed by an arbitrary finite-index subgroup of the Symmetry Group. Acta Crystallographica Section A Foundations and Advances, 79(6): p.558.)
Imagine yourself standing on a concrete floor. Your goal is to completely cover the floor with red and blue square tiles, but in a way that follows three rules:
1) Every time you step on a tileless area, you must lay a tile. You can place either a red or blue tile on your starting position.
2) When you move one step horizontally or vertically, you must place a tile that is not the same color as the one you left. For example, if you started on a red tile and moved right, left, up, or down, lay a blue tile on your new location.
3) When you move one step diagonally, you must place a tile with a similar color to the tile you left. If you were standing on a red tile and moved diagonally, lay a red tile.
Once you have covered the floor with tiles, you will have created a colored checkerboard pattern. More interestingly, you will have also created a 2D model for the structure of salt crystals, which consists of alternating sodium and chlorine atoms: Red tiles can represent sodium atoms, while blue tiles can represent chlorine atoms.
Coloring a pattern such as the checkerboard is simple, but it becomes more complicated as patterns become more intricate. Dr. Allan Junio of the UP Diliman College of Science Institute of Mathematics (UPD-CS IM) created a technique that systematically colors patterns, helping model the structures of crystals.
Much like the three-step process in tiling the floor, Dr. Junio’s technique involves a set of general mathematical rules, called theorems and lemmas, in coloring different patterns. Moreover, his method enumerates all possible ways a pattern can be colored while limiting the number of colors used.
“The reason for this restriction is that we will be applying the coloring framework on the atoms of crystal structures, where the colors correspond to exactly one kind of atom, and we will be dealing with crystal structures with only finitely many kinds of atoms,” Dr. Junio explained in his paper.
The technique can help model a process called ordered substitution, where a group of atoms are replaced with a different element, thereby deriving a new material.
To demonstrate, he applied his technique to sphalerite crystal – a mineral that is difficult to identify due to its similarity with other crystals, hence its name coming from the Greek word sphaleros, meaning “treacherous.” Sphalerite is commonly made up of zinc and sulfur atoms linked together in a pattern.
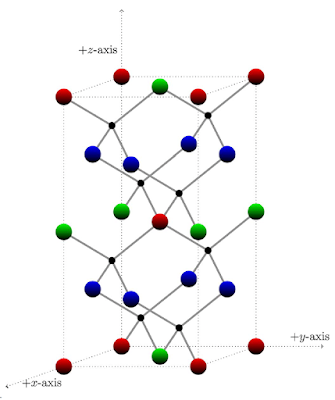
Model for the structure of sphalerite. Zinc atoms are colored green, red, and blue, while sulfur atoms are colored black. (Photo credit: Junio, A. O. Colorings of patterns fixed by an arbitrary finite-index subgroup of the Symmetry Group. Acta Crystallographica Section A Foundations and Advances, 79(6): p.555.)
Instead of representing all zinc atoms with only one color, he colored different groups with green, red, and blue. In doing so, ordered substitution becomes simpler: by replacing the blue zinc atoms with copper atoms, the green zinc atoms with iron atoms, and the red zinc atoms with tin atoms, a new material called stannite is produced. Other crystals such as kuramite and chalcopyrite can also be produced using a similar process.
Although only sphalerite and its derivatives are modeled in the paper, the technique can be applied to other crystals as well.
“It may be a good idea to determine whether the resulting colorings correspond to known compounds, and if no such compounds exist, to establish whether the colorings may be realized as physical structures using chemical and molecular properties and restrictions,” Dr. Junio concluded in his paper, which is now published in Acta Crystallographica.
Imagine yourself standing on a concrete floor. Your goal is to completely cover the floor with red and blue square tiles, but in a way that follows three rules:
1) Every time you step on a tileless area, you must lay a tile. You can place either a red or blue tile on your starting position.
2) When you move one step horizontally or vertically, you must place a tile that is not the same color as the one you left. For example, if you started on a red tile and moved right, left, up, or down, lay a blue tile on your new location.
3) When you move one step diagonally, you must place a tile with a similar color to the tile you left. If you were standing on a red tile and moved diagonally, lay a red tile.
Once you have covered the floor with tiles, you will have created a colored checkerboard pattern. More interestingly, you will have also created a 2D model for the structure of salt crystals, which consists of alternating sodium and chlorine atoms: Red tiles can represent sodium atoms, while blue tiles can represent chlorine atoms.
Coloring a pattern such as the checkerboard is simple, but it becomes more complicated as patterns become more intricate. Dr. Allan Junio of the UP Diliman College of Science Institute of Mathematics (UPD-CS IM) created a technique that systematically colors patterns, helping model the structures of crystals.
Much like the three-step process in tiling the floor, Dr. Junio’s technique involves a set of general mathematical rules, called theorems and lemmas, in coloring different patterns. Moreover, his method enumerates all possible ways a pattern can be colored while limiting the number of colors used.
“The reason for this restriction is that we will be applying the coloring framework on the atoms of crystal structures, where the colors correspond to exactly one kind of atom, and we will be dealing with crystal structures with only finitely many kinds of atoms,” Dr. Junio explained in his paper.
The technique can help model a process called ordered substitution, where a group of atoms are replaced with a different element, thereby deriving a new material.
To demonstrate, he applied his technique to sphalerite crystal – a mineral that is difficult to identify due to its similarity with other crystals, hence its name coming from the Greek word sphaleros, meaning “treacherous.” Sphalerite is commonly made up of zinc and sulfur atoms linked together in a pattern.
Model for the structure of sphalerite. Zinc atoms are colored green, red, and blue, while sulfur atoms are colored black. (Photo credit: Junio, A. O. Colorings of patterns fixed by an arbitrary finite-index subgroup of the Symmetry Group. Acta Crystallographica Section A Foundations and Advances, 79(6): p.555.)
Instead of representing all zinc atoms with only one color, he colored different groups with green, red, and blue. In doing so, ordered substitution becomes simpler: by replacing the blue zinc atoms with copper atoms, the green zinc atoms with iron atoms, and the red zinc atoms with tin atoms, a new material called stannite is produced. Other crystals such as kuramite and chalcopyrite can also be produced using a similar process.
Although only sphalerite and its derivatives are modeled in the paper, the technique can be applied to other crystals as well.
“It may be a good idea to determine whether the resulting colorings correspond to known compounds, and if no such compounds exist, to establish whether the colorings may be realized as physical structures using chemical and molecular properties and restrictions,” Dr. Junio concluded in his paper, which is now published in Acta Crystallographica.
By Harvey Sapigao
























 Ross is known as the Pambansang Blogger ng Pilipinas - An Information and Communication Technology (ICT) Professional by profession and a Social Media Evangelist by heart.
Ross is known as the Pambansang Blogger ng Pilipinas - An Information and Communication Technology (ICT) Professional by profession and a Social Media Evangelist by heart.








.jpg)



The article highlights an innovative educational approach, merging art and mathematics through "paint by numbers" activities. This method engages learners by blending creativity with logical thinking, fostering both artistic expression and mathematical skills. The trend reflects the growing popularity of online coloring resources like those offered by ColorearW.net, where users can freely download and print PDF templates, supporting interactive learning experiences.
ReplyDeleteCombining art and education is both nurturing and empowering. In a world where creativity meets learning, her coloring pages offer children a chance to express themselves freely. With easy-to-download and print PDFs at SSColoring.com, kids can explore art in a safe, welcoming environment, fostering both creativity and confidence.
ReplyDeleteHave you ever mixed mathematics with art? Dr. Allan Junio's method for coloring crystal structures is like a scientific paint-by-numbers, turning complex patterns into vibrant models. This approach could inspire online coloring enthusiasts to explore free, printable PDFs at ColorearW.com, blending creativity with curiosity.
ReplyDeleteThis fascinating review of a UP mathematician’s work on modeling crystal structures highlights the power of mathematics in scientific discovery. The innovative rules for crystal formation enhance our understanding of materials science, with potential real-world applications. A brilliant blend of math and chemistry that showcases groundbreaking research in the field!
ReplyDeleteFile for Divorce New York
This article was such an inspiring read! The creative use of visual models in science reminds me of how kids learn through colors. For something fun this spring, search for “lente kleurplaat” or visit: azkleur.nl/kleurplaat-lente/
ReplyDeleteThis article is impressive and showcases a creative intersection between mathematics and materials science. Using coloring rules to model crystal structures not only provides a visual approach but also opens up new directions for applied research in materials engineering.
ReplyDeleteFrom models like these, I believe researchers and educators can also explore the use of sound to make scientific presentations more engaging. A simple yet fun tool is the memes soundboard ( visit: memesounds.net ), which offers a collection of short sound effects—great for highlighting key moments during lectures or presentations.